Pharmaceuticals are meant to help patients become healthier. This requires consistent, high quality active pharmaceutical ingredients (APIs), responsible for the therapeutic benefits and which are formulated in the final medicinal products. Hence, potential impurities in the APIs are to be kept as low as possible, as they are most often toxic compounds.
For the related impurities, 3 thresholds are given in the European Pharmacopoeia monograph 2034 (substances for pharmaceutical use), based on the ICH guidelines: impurities above any of these thresholds require an action, which can be (1) to report, (2) to identify or (3) to toxicologically qualify them. The table below gives these thresholds, which are based on the maximum daily dose, and which are expressed in percentage and/or in quantity for the identification and qualification thresholds.
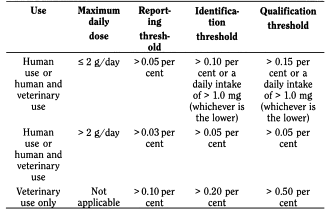
Assignment
Compute the reporting, identification and qualification thresholds of organic impurities in active substances for a given maximum daily dose.
Input
A maximal daily dose (float) expressed in grams.
Output
The reporting, identification and qualification thresholds of organic impurities in active substances for the maximum daily dose given in the input. Stick to the formatting specification of the example output below. Percentages should always be expressed with four decimal digits.
Example
Input:
0.5Output:
reporting threshold: 0.0500%
identification threshold: 0.1000%
qualification threshold: 0.1500%